Effect of cooling and heating temperature cycle during emulsification
Emulsification is the effect of a liquid in which even tiny droplets are evenly dispersed in another liquid that is incompatible with each other. Emulsification is a liquid-liquid interface phenomenon. Two immiscible liquids, such as oil and water, are divided into two layers in a container. Oil with a lower density is in the upper layer, and water with a higher density is in the lower layer. If an appropriate surfactant is added and the mixture is vigorously stirred, the oil is dispersed in water to form an emulsion. This process is called emulsification. They usually involve reducing the oil solubility in the continuous medium once by changing the temperature or solvent or phase change, during which the preferred curvature of the surfactant layer changes its sign. Here, we use a narrow range of temperature cycles to repeatedly break up the droplets into a high-energy state. (Emulsification kettle of customer’s production workshop)
(Emulsification kettle of customer’s production workshop)
The amount of emulsion processed in the world is 100 million tons per year, and the energy efficiency of the classic emulsification process is very low, which is less than 0.01% for microdroplets. For sub-micron devices, the efficiency is even lower, because the use of high shear and / or high pressure mechanical equipment will generate high temperatures, which will lead to further incompatibility with many drugs, cosmetics and food ingredients.
For non-polar oils with larger molecules, it is often encountered in practice that phase inversion self-emulsification technology is developed. This method relies on changes in temperature or surfactant concentration to change the preferred interface curvature. The principle of the self-emulsification method is through a temperature cycle between freezing and subsequent melting of the droplets in the coarse emulsion, causing the droplets to spontaneously break. Most of the usual emulsification methods are to heat the external phase and internal phase to about 80°C (75°C-90°C) for emulsification, and then to stir and cool, which consumes a lot of energy in this process.
The emulsification temperature has a great influence on the quality of emulsification, but there is no strict limit on the temperature. For example, if both oil and water are liquid, it can be emulsified by stirring at room temperature. The general emulsification temperature depends on the melting point of the high-melting point substance contained in the two phases, and factors such as the type of emulsifier and the solubility of the oil phase and the water phase should also be considered. In addition, the temperature of the two phases should be kept nearly the same, especially when emulsifying wax and fatty oil phase components with higher melting points (above 70°C), the low-temperature aqueous phase cannot be added to prevent wax, The fat crystals are precipitated, resulting in massive or coarse uneven emulsion. Generally, when emulsifying, the temperature of the oil and water phases can be controlled between 75°C -85°C. If the oil phase has high melting point wax and other components, the emulsification temperature will be higher at this time. In addition, if the viscosity increases greatly during the emulsification process, if it is too thick and affects the stirring, then some emulsification temperature can be increased appropriately. If the emulsifier used has a certain phase inversion temperature, the emulsification temperature is also preferably selected around the phase inversion temperature. The emulsification temperature sometimes has an effect on the particle size of the emulsion. For example, when fatty acid soap anionic emulsifier is generally used for emulsification with the nascent soap method, the emulsification temperature is controlled at 80 ° C, and the emulsion particle size is about 1.8-2.0 μm. If emulsification is performed at 60°C, the particle size is about 6 μm. When emulsified with a non-ionic emulsifier, the emulsification temperature has a weak influence on the particle size.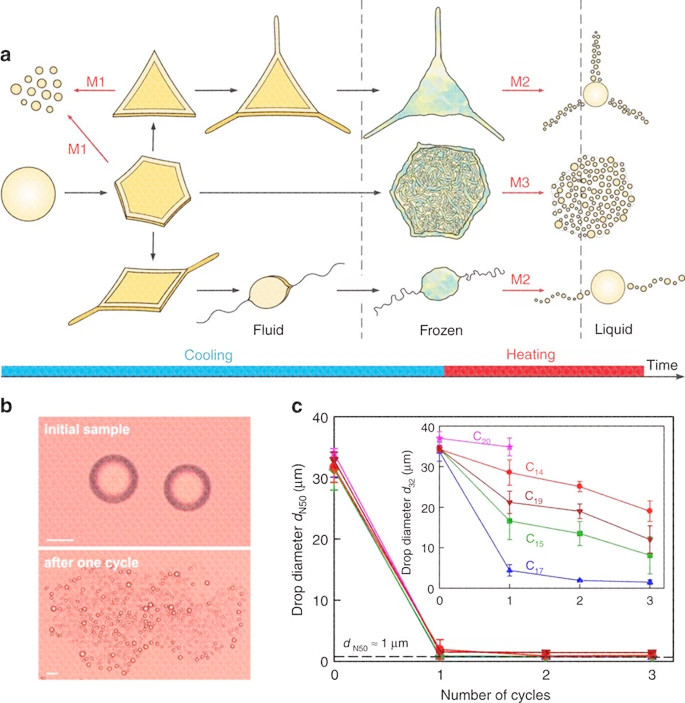
As shown in the figure above, a When cooling and heating the dispersed alkane droplets (left to right), the main droplet shape transition 20 and the related droplet breakup mechanism (M1-3, red arrow). In M1, the droplets spontaneously rupture during cooling, while in M2 and M3, the frozen particles rupture when they melt. b Microscope images of droplets in pentadecane emulsion stabilized by 1.5 wt% C 16 SorbEO 20 surfactant before and after the cooling / heating cycle (scale bar, 20 μm). c The typical change in the two measurements of the average droplet diameter d N50 and d 32 is a function of the number of F / T cycles of the C 18 EO 20 stabilized alkane in water emulsion. The cooling rate is 0.2Kmin -1, and the heating rate is 1.6Kmin -1.
The use of self-emulsification process to develop new technologies is of great potential significance for the production of temperature-sensitive drug emulsions and dispersions. For example, the self-emulsification of bulk emulsion: A 15 ml sample was placed in a glass container to study the evolution of droplet size in bulk emulsion. First cool these samples in the refrigerator at a temperature of 7°C for 2 hours to achieve complete freezing of the oil droplets, and then melt the emulsion droplets by placing the container at 25°C. Within the first 20 minutes, the initial cooling and heating rate of the emulsion was measured to be ≈0.4 K min -1, and then gradually decreased to zero as the sample temperature approached the ambient temperature. (Heating and cooling cycle equipment of emulsification kettle)
(Heating and cooling cycle equipment of emulsification kettle)
LNEYA dynamic temperature system SUNDI series temperature control range from 120°C ~ 350°C, high precision and smart, high cooling power of 0.5 to 1200kW, with production stability and repeatability. Use plate heat exchangers and tube heaters to increase heating and cooling rates. The ultra-high temperature cooling technology can directly cool from the high temperature of 300 degrees. It does not evaporate any heat transfer medium at high temperature, and can achieve -80°C~ 190°C, -70°C~ 220°C, -88°C~ 170 ℃, -55°C ~ 250°C, -30°C ~ 300°C continuous temperature control. In the emulsification reaction process, it not only saves labor costs for customers, but also improves the quality and production efficiency of products.
(The content in this article is borrowed from the Internet. If you have any questions, please contact sales@lneya.com)
 LNEYA Industrial Chillers Manufacturer Supplier -
LNEYA Industrial Chillers Manufacturer Supplier -











